10 Preparing Different Types of Compounds
Pharmacy compounding involves a wide variety of techniques used to prepare customized medications for individual patients. From simple solutions to complex emulsions and semi-solids, each dosage form requires specific handling, tools, and preparation steps to ensure uniformity, stability, and therapeutic effectiveness.
This chapter provides practical, step-by-step guidance on the general procedures used to prepare a variety of compounded formulations, including ointments, creams, pastes, solutions, suspensions, eutectic mixtures, emulsions, and capsules. While not exhaustive, these procedures are designed to serve as foundational templates when developing Master Formulation Records and Compounding Records.
How to incorporate a powder or liquid into an ointment or cream:
- Gather all ingredients and appropriate equipment
- Weigh, measure and label all ingredients
- If necessary, triturate solid ingredients into a fine powder using a mortar and pestle
- Levigate a small amount of the base ointment or cream with the powder or liquid to form a uniform, smooth paste **SEE NOTE BELOW**
- Using the principles of geometric dilution, gradually add more base to the paste, levigating after each addition to ensure uniform distribution
- Weigh empty ointment pot, transfer into ointment pot and calculate yield
- Package and label, including BUD
**NOTE** – A levigating agent may be needed, such as mineral oil, to make a uniform, smooth paste, before incorporation into the base
Watch video on how to incorporate a powder into a cream:
How to incorporate a powder into an ointment to create a paste:
- Gather all ingredients and appropriate equipment.
- Weigh, measure and label all ingredients.
- If necessary, triturate solid ingredients into a fine powder using a mortar and pestle.
- Transfer powder to the middle of the glass slab. If there are more than one powder, the powders can be combined together.
- Transfer the ointment base to the top corner of the glass slab.
- Levigate a small portion of ointment into the powder with a spatula. Spatulate until a smooth thick paste is formed.
- Once all of the powder is incorporated into the ointment, incorporate any remaining ointment by geometric dilution.
- Weigh empty ointment pot.
- Transfer the paste into the ointment pot.
- Weigh final product and calculate yield and complete quality assurance checks.
- Label and ensure pharmaceutical elegance.
How to compound a solution:
- Gather all ingredients and appropriate equipment.
- Calibrate prescription oval if necessary.
- Measure the solvent. Add solutes such as APIs, preservatives, sweeteners, and flavoring agents to the solvent in the correct order.
- Stir to ensure complete dissolution. Heat may be necessary, for instance, gently warming for poorly soluble drugs. *if using heat, ensure you know the melting point of the API to prevent destruction of the API.
- Some drugs may require pH adjustment for solubility or stability.
- Once all solutes are fully dissolved, bring the solution to final volume.
- Mix thoroughly to ensure homogeneity.
- Filter solution if needed to remove undissolved particles or to clarify the solution.
- Package and label, including storage and beyond use dating.
How to compound a suspension:
- Gather all ingredients and equipment required.
- Calibrate prescription oval to final volume.
- Triturate the active ingredient if required using mortar and pestle.
- Place powder into mortar. Wet the powder with a small amount of wetting agent or vehicle. Levigate to make a smooth, homogenous paste.
- Add a small amount of vehicle (suspending agent) to the paste and mix thoroughly. Geometric dilution is applicable here but only until liquid is formed. Continue adding the suspending vehicle slowly until mixture is pourable.
- Transfer mixture to the calibrated oval.
- Rinse mortar with small amounts of vehicle and pour rinsings into oval (two to three rinsings is standard practice to ensure all drug is transferred into final suspension).
- Qs to final volume with the vehicle.
- Mix well by shaking to ensure uniform distribution.
- Label. Apply appropriate auxiliary label “Shake well before use”, BUD, and storage instructions.
NOTE:
Common vehicles used in suspensions traditionally include simple syrup and methylcellulose. While simple syrup can be made at the pharmacy from sugar and water, it is readily available and usually bought as a ready-made product. Today, it’s more commonplace to use commercially available suspending agents such as Ora-Plus, Ora-Sweet, Ora-Blend and Suspendit. These commercially available suspending agents are pre-formulated to simply preparation and enhance consistency, stability, and palatability of the final compound and are widely used to create stable, uniform suspensions of insoluble drugs.
Watch video on how to make a Suspension:
How to compound a eutectic mixture:
A eutectic mixture is a combination of two or more solid substances that, when mixed, melt at a lower temperature than either component alone. This occurs due to molecular interactions that disrupt the crystalline structure of the individual components, leading to a decrease in the melting point.
- Forms a liquid or soft mass upon mixing, even at room temperature.
- Common in powder or topical compounding, where careful handling is needed to prevent liquefaction.
- Example: Camphor & menthol → Used in topical analgesic preparations.
- Gather all ingredients and equipment required
- Weigh all ingredients
- Using a glass slab, press crystals together with a spatula (spatulate) to reduce particle size
- The components will gradually liquefy as their melting points are depressed
- Continue spatulation until a homogeneous, smooth liquid is formed
- Using the principles of geometric dilution, incorporate liquid into a base for patient use
- Transfer to ointment pot and label, including BUD and storage
NOTE:
Forming a eutectic mixture requires a certain ratio (by weight) of the individual ingredients. For instance, camphor and menthol form a eutectic mixture in approximately a 1:1 ratio. Lidocaine and prilocaine form a eutectic mixture in a 1:1 ratio (available in the commercially available EMLA cream).
Watch video on compounding a eutectic mixture:
How to compound an emulsion:
1. Select the Emulsification Method
There are three main methods for preparing a primary emulsion:
1. Dry Gum (Continental) Method (Common for O/W emulsions)
-
- Ratio: 4 parts oil : 2 parts water : 1 part emulsifier (e.g., acacia).
- Procedure:
- Weigh and mix the emulsifying agent (e.g., acacia) with the oil phase in a dry mortar.
- Add all the water at once and triturate rapidly until a creamy white emulsion forms.
- Dilute with additional water while continuing to mix.
2. Wet Gum (English) Method (Common for O/W emulsions)
-
- Ratio: Same as Dry Gum (4:2:1).
- Procedure:
- Mix the emulsifier with water first to form a mucilage.
- Slowly add oil in small portions, triturating after each addition until a stable emulsion forms.
- Continue mixing while adding more liquid as needed.
3. Bottle (Forbes) Method (For volatile oils or small-volume emulsions)
-
- Procedure:
- Place emulsifying agent and oil in a dry bottle.
- Shake vigorously, then gradually add water in portions, shaking well after each addition.
- Procedure:
2. Homogenization and Mixing
-
- Once the primary emulsion is formed, further homogenization can be done using:
- Mechanical blenders (for large-scale emulsions).
- Hand stirring (for small-scale pharmacy preparations).
- Once the primary emulsion is formed, further homogenization can be done using:
3. Adjusting Stability
-
- Add stabilizers or viscosity enhancers (e.g., gelatin, tragacanth) if needed.
- Adjust pH with appropriate buffers to enhance stability.
4. Finalizing the Emulsion
-
- Transfer to a suitable container (e.g., amber bottle for light-sensitive emulsions).
- Label with storage conditions (e.g., “Shake well before use”).
- Determine Beyond-Use Date (BUD) according to USP guidelines.
How to compound capsules:
- Gather all ingredients and equipment required
- Select the capsule size (smallest size capable of holding the formulation in the capsule body)
- Prepare capsule formulation (determine if fillers are required, aliquot may be needed)
- Fill capsule using punch method (Hand Method – see below) or with capsule filling machine (Machine Method – see below)
- Use finishing procedures (cleaning, polishing)
- Package
- Check physical appearance (colour, uniformity, extent of fill, ensure capsules are locked if they have a locking feature)
- Label including BUD
- Hand method:
- Count out exact number of capsules required
- Triturate API if necessary, and combine powders using geometric dilution
- Place powder on slab
- Smooth and block powder with a spatula to 1/3 or 1/2 length of capsule body
- Tare scale with weigh paper and an empty capsule
- Repeatedly punch and rotate the open end of the capsule until it is filled
- Place cap on body of capsule
- Weigh each capsule and add or remove powder until correct weight is achieved
- Finish capsule (clean and polish)
- Calculate % deviation
- Machine method:
- Load hard capsules into machine
- Tighten the plate screws around the capsule bodies, then lift
- Loosen the plate so the bodies so they align with the surface of the machine
- Pour the prepared powder onto the plate and spread evenly into each capsule body
- Ramp down with comb
- Repeat (usually twice) until the capsules are full
- Replace the caps onto the capsule body
- Hand method:
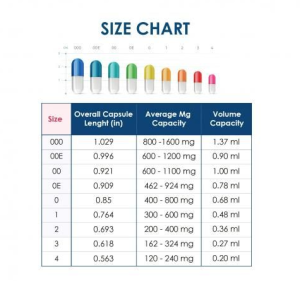
The safe and effective preparation of compounded products relies on consistent technique, attention to detail, and a solid understanding of the properties and requirements of each dosage form. While these procedures are general in nature, they serve as a reliable starting point when preparing individualized formulations. Practitioners must always refer to current compounding standards, regulatory expectations, and specific formulation references when adapting or expanding upon these methods. Ultimately, each preparation must meet the therapeutic needs of the patient, uphold professional standards, and comply with relevant compounding guidelines.
Reference: Shrewsbury, R. P. (2020). Applied pharmaceutics in contemporary compounding (4th ed.). Morton Publishing Company.

