21 Importance of Visual Inspection
Visual verification is such an essential step in the compounding process, serving as a frontline safeguard to ensure that compounded preparations meet established standards for quality, safety, and consistency before they reach the patient.
During visual verification, pharmacy personnel carefully inspect the final product for physical characteristics such as color, clarity, absence of particulates, uniformity, and signs of contamination or instability (e.g., precipitation, separation, or unexpected cloudiness). This inspection also includes checking the integrity of the container and closure system, ensuring there are no leaks, cracks, or defects that could compromise the product. By comparing the compounded product to the documented formulation standards and compounding records, the verifier can confirm that the correct ingredients and quantities have been used, and that the preparation process was followed precisely. Visual checks are also used to verify accurate labeling, including patient information, drug name and strength, beyond-use date, and any required auxiliary labels.
These are some of the important things to check the final preparation for:
- Colour
- Look at the Master Formulation Record to verify the preparation looks as is expected.
- This can often give you a hint as to whether the compound has all the correct ingredients.
- It can also be a clue if there is an ingredient that isn’t up to necessary quality, or has gone bad.
- Smell
- As above, the smell can indicate if the correct ingredients have been used or if there is an ingredient that has gone bad.
- Quality (no streaks, lumps, contaminants)
- There should be no streaks, lumps or contaminants (specks or particulate in the product).
- Quantity
- If you are supposed to have a certain amount of the preparation and there is a significantly more or less that is expected, chances are an incorrect quantity was used.
- There should be an expected yield listed in the MFR that can guide your final verification. Yields can vary based on the preparation. For instance, little loss occurs when compounding creams and ointments, but more loss occurs when making suppositories. When you are qs’ing to final volume, expected yield is 100%.
- Label (including auxiliary labels)
- The label must include the BUD, and special storage conditions and the prescription label with all of the legal requirements of a prescription label.
- Packaging
- Ensure the correct size and type of packaging is used.
- Pharmaceutical elegance
- The final product should be clean on the outside. There should be no finger prints, smudges or any compound on the outside of the container.
- Contaminants
- A number of things could be in the final preparation such as dirt, hair, a bug, a finger nail etc.
Examples of Unsuitable Compounds:
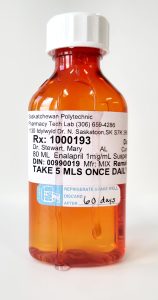
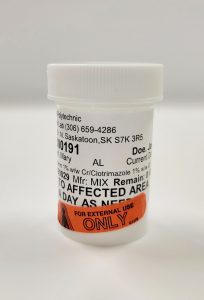

In order to verify the preparation is ready to be dispensed, you must open the lid and look inside. If the preparation is in a prescription oval, look through the sides and at the bottom.
**If anything appears questionable—whether it’s cloudiness, the wrong color, the incorrect volume or weight, or unidentified particles—the product should be set aside, documented, and not dispensed until reassessed or remade.
The importance of visual verification lies in its ability to detect errors or inconsistencies that might not be apparent from documentation alone, such as accidental contamination, incorrect mixing, or physical instability that could affect safety or efficacy. By systematically performing and documenting visual inspections, pharmacies reduce the risk of dispensing compromised or unsafe products, support regulatory compliance, and reinforce a culture of quality assurance in compounding practice.

