25 Risk Assessment and the Use of Personal Protective Equipment
Understanding the key roles in compounding supervision is an important part to ensuring the safe, effective, and compliant preparation of non-sterile compounded products. To ensure the quality of compounded products and equally as important, to protect personnel, a risk assessment must be performed for each non-sterile preparation. This assessment helps ensure appropriate safety measures, including PPE, are selected and applied.
Risk assessment evaluates:
- Properties of the active pharmaceutical ingredient (API)
(e.g., toxicity, irritant properties, carcinogenicity, reproductive toxicity) - Dosage form and route of administration
(e.g., topical, oral, mucosal) - Concentration of hazardous substances
- Potential for personnel exposure
(e.g., inhalation, skin contact, ingestion) - Complexity of compounding process
(e.g., need for special manipulations, open handling - Frequency and volume of compounding
- Exposure risk to staff and cumulative exposure over time
- Risk of cross-contamination (e.g., allergens)
- WHMIS classification or NIOSH hazardous drug classification
- Microbial contamination risk (especially in creams, ointments, liquids)
- Education and competency of compounding staff
- Adequacy of equipment and facilities
Risk levels (NAPRA Classification):
- Simple: Routine procedures with little risk (e.g., mixing creams together).
- Moderate: Requires special calculations or techniques (e.g., powders mixed with topical creams, suspensions).
- Complex: Requires specialized training, facilities, or equipment (e.g., hormone, chemotherapy drug preparations).
Outcome of the risk assessment determines:
- Necessary PPE
- Facility requirements (e.g., use of containment primary engineering controls, C-PECs)
- Handling, storage, and disposal procedures
In addition to evaluating individual preparations, the overall or cumulative risk of all compounded products in the pharmacy must be considered. For example, while a single low-risk preparation may not require extensive measures, multiple low-risk products compounded in a short period may increase overall risk. These scenarios must be documented and addressed appropriately.
If the pharmacy must compound a product that involves procedures not currently in place, a risk mitigation plan must be documented. This includes identifying potential hazards, listing extra precautions (e.g., workflow controls, verification steps, or containment strategies), and citing evidence-based references supporting the effectiveness of those measures.
Examples:
- For a complex compound, specify extra requirements such as uninterrupted workflow, specialized equipment, additional checks, and supporting documentation.
- If handling hazardous substances, ensure alternative containment or safe handling practices are documented in the Master Formulation Record and that applicable safety data sheets and references are consulted.
Each pharmacy must maintain an updated list of drugs and materials used, including those classified as hazardous. All relevant federal, provincial, and territorial regulations must be reviewed to determine appropriate compounding practices.
Definition of a complex compound:
A complex compound in non-sterile compounding refers to a preparation that requires specialized knowledge, skills, equipment, or procedures beyond basic compounding techniques. These compounds often involve multiple ingredients, critical calculations, or unique administration routes, and pose higher risks if compounded incorrectly.
Key Characteristics of a Complex Compound:
- Involves multiple steps that must be precisely followed
- Requires special equipment (e.g., ointment mills, pH meters, capsule machines)
- Includes unstable or reactive ingredients
- Demands tight control of environmental conditions (e.g., temperature, humidity)
- Requires modification of dosage forms (e.g., crushing tablets, suspending powders)
- Often intended for non-standard routes of administration (e.g., rectal, vaginal, nasal)
- Requires advanced calculations for dosing or ingredient conversion
- May involve non-routine procedures, such as emulsification or sustained-release preparations
Examples:
- Transdermal gels containing hormones
- Oral suspensions for pediatric patients using modified ingredients
- Rectal suppositories that require melting and molding
- Customized topical creams with multiple APIs (active pharmaceutical ingredients)
- Nasal sprays with strict particle size requirements
Risk assessments must be reviewed annually and updated as needed.
Note: Even if hazardous compounds are used only occasionally, they must not be considered in isolation. The aggregate risk across all preparations should be evaluated and documented.
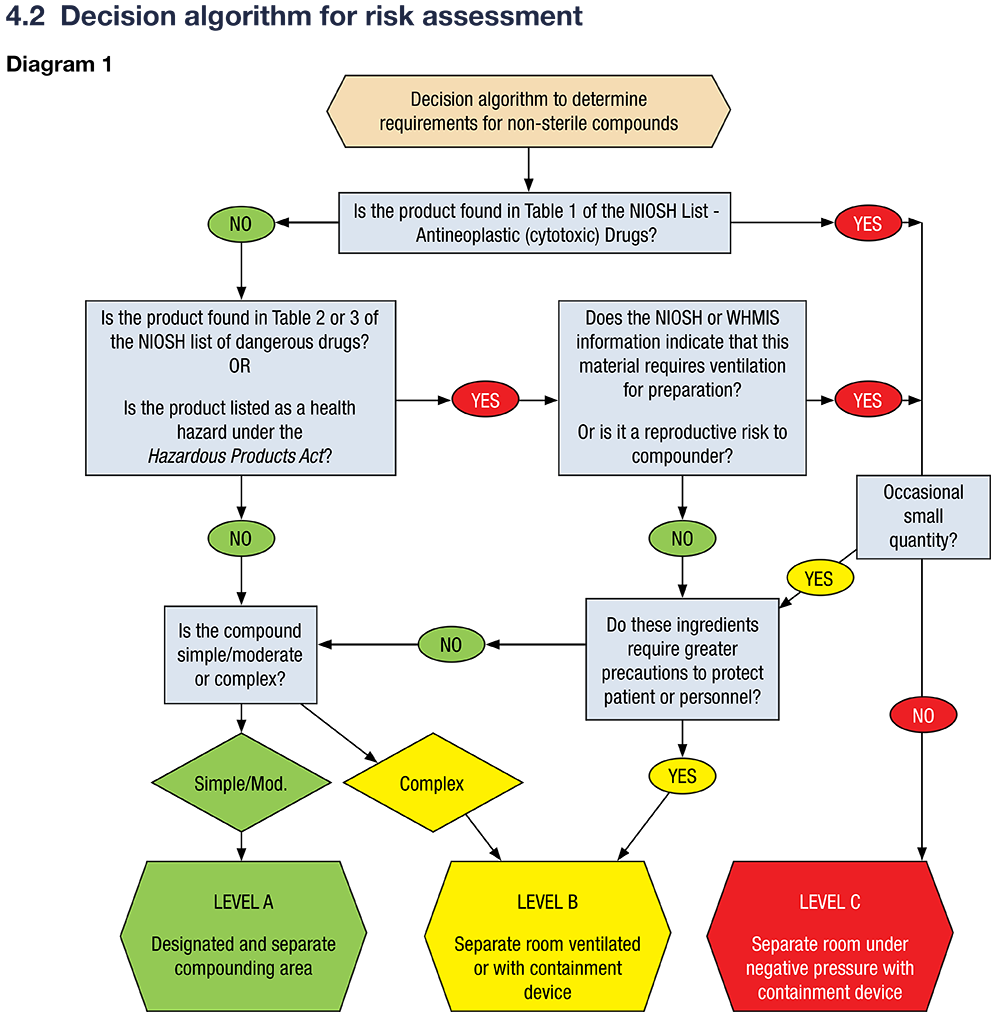
Risk Assessment Decision Tool
A decision algorithm is available to guide pharmacists in determining the appropriate risk level and facility requirements for non-sterile compounding. This algorithm considers the drug’s classification (e.g., NIOSH Tables 1–3, WHMIS), reproductive risks, and compounding complexity. As shown in a previous chapter, NAPRA has developed a decision algorithm for risk assessment:
Resources for Risk Assessment
Refer to the following sources for guidance on assessing risk and determining safe compounding practices:
- USP Compounding Compendium (USP <795>, <800>)
- CSHP Guidelines for Pharmacies
- PIC/S Good Compounding Practices
- NIOSH List of Hazardous Drugs
- Hazardous Products Act & WHMIS 2015
- WorkSafeBC Guidelines on Hazardous Drug Handling
- ASSTSAS Safe Handling of Hazardous Drugs Guide
These resources help ensure that pharmacy staff apply appropriate safety measures and follow current standards when handling hazardous or complex compounds.
The risk assessment is used to determine the NAPRA requirements for non-sterile compounding. The requirements are in divided into 3 levels, A, B and C. As you can see, the requirements are much more stringent for complex and hazardous compounds than for simple and moderate compounds.
| Level | What is Included | Requirements |
| Level A | – Simple and moderate compounds as defined by USP <795> | – Designated separate space for compounding activities – Facilities must allow for orderly storage and safe workflow – Must be cleaned and maintained to prevent contamination – Only trained personnel permitted in the area |
| Level B | – Complex compounds as defined by USP <795> – Preparations occasionally involving substances that require ventilation |
– Separate, well-ventilated room dedicated to compounding
– Sufficiently large workspace and appropriate specialized equipment
|
| Level C | – Hazardous drugs (NIOSH Group 1) – Hazardous materials (WHMIS health hazards) – Large quantities of APIs (NIOSH Group 2/3 drugs) used routinely |
– Physically separated, dedicated compounding room – Room under negative pressure relative to surrounding areas – External ventilation through HEPA-filtered exhaust – Installation and use of a Containment Primary Engineering Control (CPEC) suitable for the risk level – Must meet specific air changes per hour (ACH) requirements – Detailed documentation and risk assessments required for each preparation |
What Personal Protective Equipment (PPE) should be used?
Purpose of PPE
PPE is essential in non-sterile compounding to:
- Protects compounders from hazardous or irritating substances
- Protects the preparation from contamination
- Complies with NAPRA standards
How to Determine Appropriate PPE for Non-Sterile Compounding:
- Assess the Type of Ingredients
-
- Irritants or sensitizers → Gloves, mask/respirator, lab coat
- Hazardous drugs (e.g., hormones, chemotherapeutics) → Full PPE – Double gloves, gown, respirator or N95, full-face shield
- Allergenic ingredients (e.g., penicillin) → Same as above, plus enhanced containment
- Evaluate the Dosage Form and Process
-
- Liquids & solutions → Lab coat, gloves, splash goggles
- Powders (prone to aerosolization) → Respirator or mask, gloves, eye protection
- Semi-solids (creams, ointments) → Lab coat, gloves
- Capsules or suppositories → Gloves, lab coat; mask if powders are involved
- Consider the Route of Administration
-
- Dermal or mucosal preparations often require more PPE to prevent self-exposure due to increased risk of absorption (e.g., hormone creams)
- Follow Risk Level Classification
-
- Simple preparations → Basic PPE (gloves, lab coat)
- Moderate/Complex or → Enhanced PPE (double gloves, gown, respirator, eye protection)
- Hazardous preparations → Full NAPRA-compliant PPE (see below)
NAPRA 9.2.3: PPE Requirements for Hazardous Non-Sterile Compounding
Gloves
-
- Double gloves (ASTM D6978) for:
- Unpacking
- Compounding
- Deactivation/decontamination
- Spill management
- Worn over gown cuffs
- Change:
- Every 30 minutes
- If damaged or contaminated
- At manufacturer’s permeation limit
- Double gloves (ASTM D6978) for:
Gown
-
- Disposable, back-closing, long sleeves with fitted cuffs
- Materials: Polyethylene-coated polypropylene or similar
- Do not use: Cloth, absorbent material, or reusable lab coats
- Change:
- Every 2–3 hours
- If removed
- If contaminated
- At permeation time limit
⚠️ Never take contaminated clothing home. If policy allows laundering, it must follow strict protocols.
Head, Hair, Shoe & Sleeve Covers
-
- Disposable covers required for:
- Hair, beard/mustache, shoes
- Sleeve covers for additional protection
- Change:
- After each removal
- If contaminated
- Double shoe covers:
- Wear second pair inside the C-SEC
- Remove when exiting
- Disposable covers required for:
Respiratory Protection
-
- Surgical masks are not sufficient for hazardous drug protection
- Use:
- N95 or N100 masks (fit-tested) for airborne particles
- Full face-piece respirator or PAPR for vapors/gases or splash risk
- Change:
- Every 3.5 hours
- After removal
- If contaminated
Eye & Face Protection
-
- Use goggles + face shield or full-face respirator when:
- Working at or above eye level
- Cleaning under C-PEC surfaces
- Managing spills
- Unpacking damaged products
- Regular eyeglasses and safety glasses are not enough
- Use goggles + face shield or full-face respirator when:
- Check Organizational Policies & SDS
-
- Always review the Safety Data Sheets (SDS) for each ingredient for PPE recommendations
- Follow institutional protocols and local/regional regulations
PPE decision chart:
| Activity / Risk Level | PPE Required | Additional Notes |
| Simple non-hazardous prep (e.g., creams, solutions) |
– Gloves – Lab coat (non-absorbent, disposable or dedicated) |
Replace if contaminated |
| Powder handling (e.g., capsules, troches) |
– Gloves – Lab coat – Eye protection – Respirator (e.g., N95 if airborne particles) |
Avoid dust exposure |
| Moderate-risk prep (e.g., allergens, irritants) |
– Gloves – Lab coat – Eye protection |
Use containment if needed |
| Hazardous drug compounding (HDs per NIOSH/NAPRA) |
– Double chemotherapy gloves (ASTM D6978) – Disposable gown (back-closing, coated) – Head & hair cover – Shoe covers (double in C-SEC) – Respirator (fit-tested N95/N100 or PAPR) – Eye protection (goggles + face shield) |
Change PPE per time/use limits Never reuse disposable PPE |
| Unpacking HDs | – Double gloves – Gown – Respirator if damaged packaging suspected |
Use caution with unknown leaks |
| Cleaning / decontaminating | – Double gloves – Gown – Eye/face protection – Respirator |
Especially under C-PEC work surfaces |
| Spill cleanup | – Full PPE as for HD compounding – Use designated spill kit |
Discard all contaminated PPE after cleanup |
| Working at or above eye level | – Eye protection (goggles) – Face shield or full-face respirator |
Prevent splash/contact exposure |
When Should PPE be Changed?
NAPRA provides specific guidance on when personal protective equipment (PPE) should be changed during non-sterile compounding, particularly for hazardous drug handling. The timing is based on both safety considerations and manufacturer recommendations:
-
Gloves:
Change gloves every 30 minutes during compounding or immediately if they become torn, contaminated, or compromised. Gloves must also be changed at the manufacturer’s permeation time limit (for chemotherapy gloves, ASTM D6978 standard applies). -
Gowns:
Disposable, back-closing gowns made of polyethylene-coated polypropylene or similar materials should be changed every 2 to 3 hours or immediately if removed or contaminated. Gowns must also be replaced at the earliest of the manufacturer’s permeation time limit. -
Respirators (e.g., N95 or N100 masks):
Respirators should be changed every 3.5 hours, after removal, or if contaminated. Surgical masks are not sufficient for hazardous drug protection. -
Head, Hair, Shoe, and Sleeve Covers:
These should be changed after each removal or if contaminated. -
Whenever the PPE becomes contaminated or compromised. For example, if gloves become torn, soiled, or come into contact with hazardous materials or non-sterile surfaces, they must be replaced immediately.
-
Between different compounding activities, especially when moving from handling hazardous to non-hazardous preparations. This prevents cross-contamination between products and ensures that hazardous residues are not transferred to other preparations or surfaces.
-
At the end of each compounding session or before leaving the compounding area. PPE should not be worn outside the compounding area to avoid spreading contaminants to other parts of the pharmacy.
Effective risk assessment in non-sterile compounding is essential to identify hazards, implement controls, and ensure safe and high-quality preparations. By evaluating factors such as ingredient properties, dosage forms, and exposure risks, pharmacies can assign appropriate risk levels and adopt safeguards ranging from basic PPE to specialized engineering controls. Importantly, risk is not static—assessments must be reviewed regularly and updated as compounding practices, formulations, or regulations evolve. This proactive approach enables pharmacies to maintain a culture of safety and continuous improvement, reducing errors and protecting both patients and staff.

