5 Risk Assessment
Risk assessment is not a one-time activity—it is a systematic, ongoing process that ensures the safety and quality of non-sterile compounded preparations. Each compounded product must be assessed individually, with the highest identified risk level guiding the pharmacy’s operational standards. Proper documentation, clear roles and responsibilities, and regular review and updating are essential to staying compliant and protecting both patients and compounding personnel.
Key Principles of Risk Assessment
- Mandatory for Each Preparation:
- Every non-sterile compounded product must undergo a risk assessment covering both risk to the preparation (e.g., contamination, stability) and risk to personnel (e.g., exposure to hazardous ingredients).
- Regular Review:
- Risk assessments must be reviewed at least annually, or sooner if there are changes in practice, standards, or the compounding environment.
- Documentation:
- All findings and decisions from the risk assessment must be documented, including references such as Safety Data Sheets (SDS), Master Formulation Records, and relevant literature.
Steps in Conducting a Risk Assessment
1. Identify the Preparation and Process
- Determine what non-sterile compounded preparations and services are being provided.
- Assign a compounding supervisor responsible for overseeing risk assessments and compliance.
2. Assess Risks to the Preparation
- Complexity of Compounding:
- Evaluate the preparation’s complexity (simple, moderate, complex).
- Potential for Contamination:
- Consider the risk of microbial, chemical, or physical contamination.
- Cross-Contamination:
- Assess the risk of cross-contamination with other products, especially allergens or hazardous substances.
- Stability and Storage:
- Consider the stability of the preparation and the appropriateness of the beyond-use date (BUD).
3. Assess Risks to Personnel
- Ingredient Hazards:
- Review the toxicity, concentration, and physical form (e.g., powder, liquid) of each ingredient.
- Exposure Potential:
- Use Safety Data Sheets (SDS), NIOSH lists, and WHMIS information to determine exposure risks.
- Required Controls:
- Identify the need for engineering controls (e.g., ventilation), personal protective equipment (PPE), and safe handling procedures.
4. Determine Level of Requirements
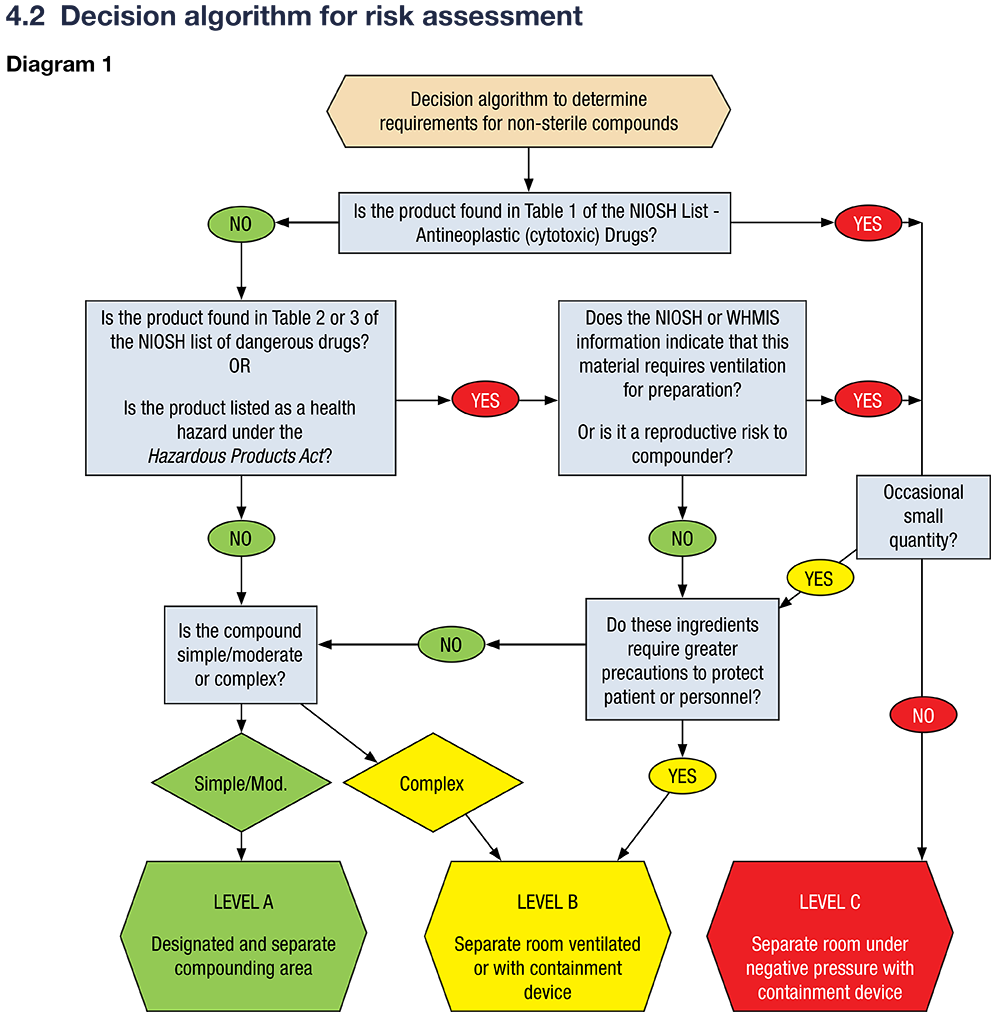
- Use the NAPRA decision algorithm (Guidance Document Section 4.2) to classify the preparation as low, moderate, or high risk, which dictates the level of facility, equipment, and procedural controls required.
- Ensure all risk mitigation strategies are in place and documented.
5. Review and Update
-
Reassess risk annually or when significant changes occur (e.g., new ingredients, updated procedures, changes in staff competency).
Factors to Consider in Risk Assessment
- When conducting a risk assessment for non-sterile compounding in Canada, several factors need to be considered to ensure safety and compliance. Here are the key factors:
- Complexity of Compounding: The complexity of the preparation process, including the number of steps and the potential for errors
- Active Pharmaceutical Ingredients (APIs): The toxicity, potency, and potential hazards of the APIs used in the preparation
- Frequency of Compounding: How often the compound is prepared, which can affect the level of risk due to repeated exposure
- Quantity of Ingredients: The amount of each ingredient being handled, as larger quantities may pose greater risks
- Concentration of Ingredients: Higher concentrations of certain ingredients can increase the risk to the compounder
- Potential for Cross-Contamination: The risk of cross-contamination with other products, especially allergens
- Microbial Contamination: The potential for microbial contamination, particularly in preparations that are more susceptible, like creams
- Personal Protective Equipment (PPE): The type and adequacy of PPE required to protect the compounder from exposure
- Facilities and Equipment: The availability and suitability of the compounding area and equipment, including ventilation and safety measures
- Verification Steps: The need for verification steps during the compounding process to ensure accuracy and safety
Roles and Responsibilities
- Pharmacy Manager:
- Ultimately responsible for ensuring risk assessments are performed and standards are met.
- Compounding Supervisor:
- May be delegated responsibility for developing, organizing, and overseeing risk assessments and related activities.
- Compounding Personnel:
- Must follow established procedures and use appropriate controls as determined by the risk assessment.
Documentation and Quality Assurance
- All risk assessments and their outcomes must be documented and retained for inspection.
- Master Formulation Records must include safety data and risk mitigation strategies for each preparation.
- Ongoing quality assurance activities, such as training, cleaning, and maintenance, must align with the identified risk level.
Risk assessments need to be completed at least every 12 months.
How Risk Assessment Determines the Level
-
Step 1: Assess Ingredients
- Identify all active pharmaceutical ingredients (APIs) and excipients.
- Consult Safety Data Sheets (SDS), NIOSH lists, and WHMIS data to determine if any are hazardous or require special handling.
-
Step 2: Assess Complexity
- Simple or moderate compounds (e.g., basic creams, solutions) generally fall under Level A.
- Complex compounds (e.g., requiring special calculations, procedures, or lacking stability data) may require Level B.
-
Step 3: Hazard Identification
- If the preparation contains hazardous drugs (e.g., those listed in Table 1 of the NIOSH list, such as antineoplastics), it automatically requires Level C controls.
- If the product is listed as a health hazard under the Hazardous Products Act or requires special ventilation or PPE, Level B or C may be needed.
-
Step 4: Assign the Level
- Level A:
- For non-hazardous, simple/moderate compounds; standard precautions suffice.
- Level B:
- For more complex or potentially hazardous compounds, but not requiring full containment.
- Level C:
- For hazardous drugs with significant risk to personnel; requires dedicated containment and engineering controls.
- Level A:
-
Step 5: If in Doubt, Use Higher Level
- If there is uncertainty about which level to assign, the higher standard must be used to ensure safety.
As stated above, the risk levels are categorized into three main levels: A, B, and C. Each level has specific requirements to ensure safety and quality. Here’s a detailed look at each level:
Level A
-
- Description: This is the lowest risk level, involving simple compounding processes.
- Examples: Compounding simple oral suspensions, mixing two or more non-hazardous creams.
- Requirements: Basic compounding area, standard operating procedures (SOPs), and minimal personal protective equipment (PPE) like gloves and lab coats
Level B
-
- Description: This level involves moderate risk, including more complex compounding processes.
- Examples: Preparing oral capsules from bulk powders, mixing non-sterile solutions.
- Requirements: Dedicated compounding area with controlled environment, more stringent SOPs, and additional PPE such as masks and eye protection
Level C
-
- Description: This is the highest risk level, involving complex and potentially hazardous compounding processes.
- Examples: Compounding with hazardous drugs, preparing hormonal transdermal gels.
- Requirements: Separate, well-ventilated compounding room, comprehensive SOPs, and full PPE including gloves, masks, eye protection, and sometimes respirators
These risk levels help ensure that appropriate measures are taken to protect both the compounder and the patient, maintaining high standards of safety and quality in non-sterile compounding.
Key Points for Pharmacy Personnel:
- Every compounded product must be risk-assessed to determine the appropriate level (A, B, or C).
- The assigned level dictates the minimum requirements for facilities, equipment, and PPE.
- Documentation of the risk assessment and rationale is mandatory.
- Regular review and updating of risk assessments are required, especially if compounding practices or products change.
Facility Requirements for Levels A, B, and C in Non-Sterile Compounding
The NAPRA Model Standards for Non-Sterile Compounding set distinct facility requirements for Levels A, B, and C, each reflecting the level of risk identified during the risk assessment process. Here’s how they differ:
Level A: Basic Facility Requirements
-
Designated Compounding Area:
Compounding is performed in a dedicated, clearly demarcated area within the pharmacy. This area must be separated from patient care and high-traffic zones to minimize contamination risk. -
No Specialized Engineering Controls:
No requirement for dedicated rooms, ventilation, or containment devices. Standard pharmacy counters and surfaces are generally sufficient. -
Cleaning and Organization:
The area must be kept clean, organized, and free from clutter, with appropriate cleaning protocols in place. -
Access Control:
Only authorized personnel should have access during compounding activities.
Level B: Enhanced Facility Requirements
-
Separate Room:
Compounding must occur in a separate, enclosed room within the pharmacy. This room provides an additional barrier to prevent cross-contamination and exposure. -
Ventilation and Air Quality:
While not as stringent as Level C, some Level B preparations may require enhanced ventilation or local exhaust (e.g., for volatile or sensitizing ingredients). -
Surfaces and Finishes:
The room must have smooth, easily cleanable surfaces and be designed to facilitate cleaning and minimize dust and contamination. -
Access Control:
The room should be restricted to compounding personnel during use.
Level C: Highest Facility Requirements (Hazardous Compounding)
-
Dedicated, Enclosed Room:
Compounding is performed in a dedicated, enclosed room specifically designed for hazardous preparations (e.g., cytotoxic drugs). -
Negative Pressure Ventilation:
The room must be maintained under negative pressure relative to adjacent areas to prevent the escape of hazardous substances. -
Containment Devices:
Use of containment primary engineering controls (C-PECs) such as ventilated containment hoods or cabinets is required. -
Specialized Cleaning and Waste Disposal:
Enhanced cleaning protocols and specialized waste disposal systems must be in place to manage hazardous residues. -
Access Control and Signage:
Strict access control, with clear signage indicating hazardous compounding activities.
Pharmacies are not assigned a fixed level (A, B, or C) under NAPRA’s Model Standards. Instead, the level of requirements depends on the risk assessments of the specific preparations they compound. Here’s how pharmacies determine what they can compound:
-
Risk Assessment Drives Compliance
Each non-sterile compounded preparation must undergo a risk assessment to determine whether it falls under Level A, B, or C. The pharmacy must comply with the highest level required for any preparation they compound.
Example: If a pharmacy compounds both Level A (simple creams) and Level C (cytotoxic drugs), it must meet Level C facility requirements for hazardous drug compounding. -
Cumulative Risk Evaluation
Even if individual preparations are low-risk (Level A), frequent compounding of multiple preparations may elevate the pharmacy’s overall risk, requiring higher-level controls.
Who Decides?
-
Designated Manager/Compounding Supervisor: Responsible for ensuring risk assessments are completed and facilities meet required levels.
-
Pharmacy Regulatory Body (e.g., SCPP): Enforces compliance during inspections
Facility Requirements for Non-Sterile Compounding (NAPRA Levels A, B, and C)
| Level | Facility Type | Key Requirements | Typical Use Cases |
|---|---|---|---|
| Level A | Designated compounding area | – Clearly demarcated area within the pharmacy – Separated from patient care/high-traffic zones – No special ventilation or containment devices required – Clean, organized, and clutter-free |
Non-hazardous, simple or moderate-risk compounds (e.g., creams, solutions) |
| Level B | Separate, enclosed room | – Dedicated, enclosed compounding room – Smooth, cleanable surfaces – May require enhanced ventilation or local exhaust – Restricted access during compounding |
Moderate-risk or complex compounds; some hazardous ingredients requiring extra precautions |
| Level C | Dedicated, enclosed room | – Separate, dedicated room for hazardous compounding – Negative pressure ventilation – Containment primary engineering controls (e.g., ventilated hoods) – Enhanced cleaning and waste protocols – Strict access control and signage |
Hazardous drugs (e.g., cytotoxic/antineoplastic drugs), high-risk compounds |
Key Points:
-
The higher the risk (as determined by risk assessment), the more stringent the facility requirements.
-
Pharmacies must meet the facility requirements for the highest level of risk associated with any preparation they compound.
-
Proper documentation, cleaning protocols, and access controls are required at all levels.
Below is an decision algorithm to aid in understanding the process of risk assessment from the NAPRA GUIDANCE DOCUMENT FOR PHARMACY COMPOUNDING OF NON-STERILE PREPARATIONS:
NAPRA MSOP for Non-Sterile Compounding Risk Assessment Algorithm
Where can you find information to help you in a risk assessment?
- SCPP https://saskpharm.ca/document/8699/Compounding_Risk_Assessment.pdf
- ACP https://abpharmacy.ca/news/assess-your-risks/
- NAPRA https://www.napra.ca/wp-content/uploads/2018/03/NAPRA-Mdl-Stnds-Pharmacy-Compounding-Nonsterile-Preparations-Guidance-EN-March-2018-CLAR-Jan-2022.pdf
- WHMIS https://www.canada.ca/en/health-canada/services/environmental-workplace-health/occupational-health-safety/workplace-hazardous-materials-information-system.html
- USP Compounding Compendium
- Canadian Society of Hospital Pharmacists Compounding: Guidelines for pharmacies. –
- video to watch***
Risk assessment is a key part of safe and effective non-sterile compounding. It helps protect both the person making the product and the patient receiving it. Each compounded preparation must be reviewed to decide how risky it is and what safety measures are needed.
Based on the results, the pharmacy must follow the right level of precautions—Level A, B, or C—depending on the ingredients, equipment, and complexity of the preparation. Risk assessments must be done before compounding and reviewed at least once a year or if anything changes.
Good documentation, clear roles, and following proper procedures help ensure safety and quality. By using tools and guidelines from NAPRA and provincial regulators like SCPP, pharmacy staff can make informed decisions and keep compounding practices safe and compliant.

